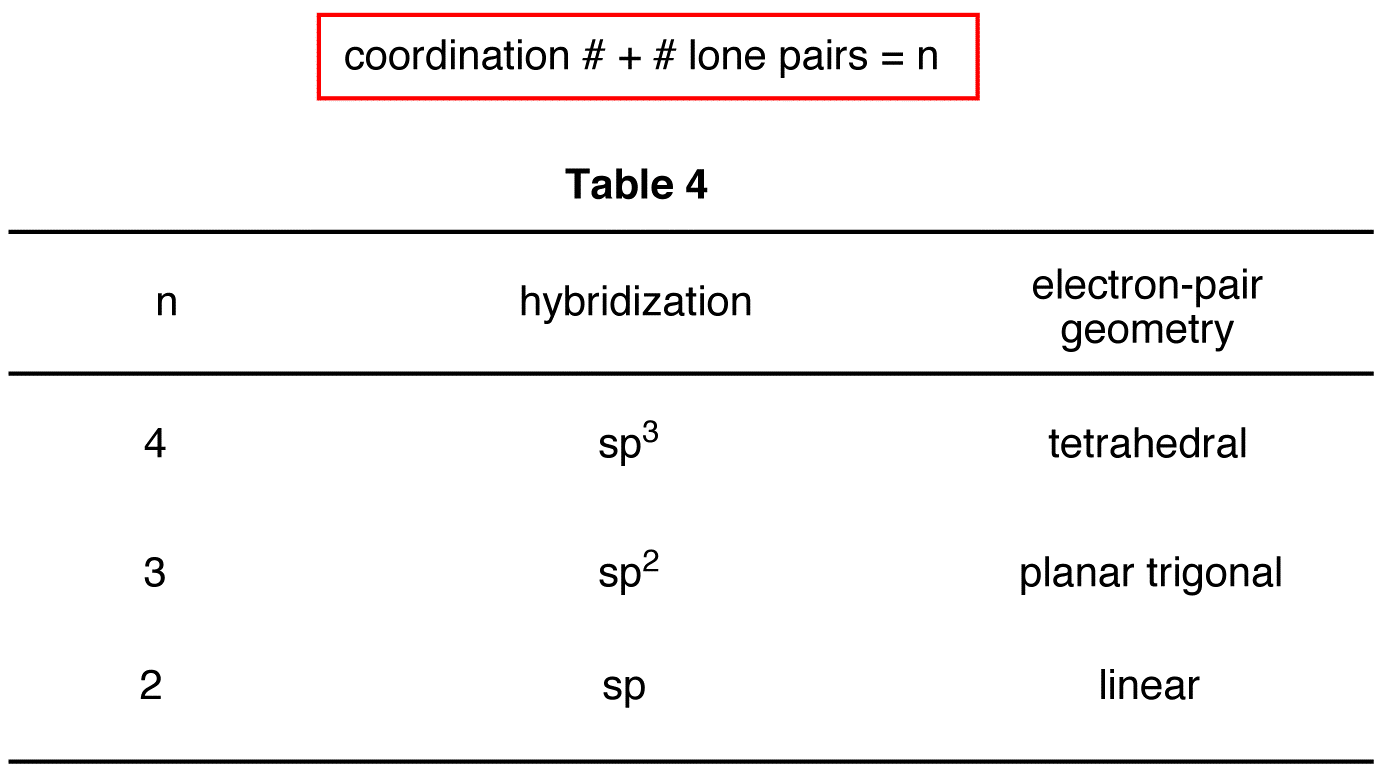
What Is Hybridization Explain Sp2 Hybridization With Example . Understand the types of hybridization, formation of new hybrid orbitals by the. A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to be linear by vsepr. The beryllium atom contains all. Web the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to. Web learn the definition of orbital hybridization and the characteristics and geometries of sp, sp2, sp3, sp3d1, and sp3d2 hybridization. Because carbon plays such a significant role in organic chemistry, we will be using it as an. Web what is hybridization? Sp 3 hybridization in methane; Web the sp2 hybridization occurs when the s orbital is mixed with only two p orbitals as opposed to the three p orbitals in. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2.
from chem.libretexts.org
Web learn the definition of orbital hybridization and the characteristics and geometries of sp, sp2, sp3, sp3d1, and sp3d2 hybridization. Understand the types of hybridization, formation of new hybrid orbitals by the. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2. A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to be linear by vsepr. Web what is hybridization? The beryllium atom contains all. Because carbon plays such a significant role in organic chemistry, we will be using it as an. Sp 3 hybridization in methane; Web the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to. Web the sp2 hybridization occurs when the s orbital is mixed with only two p orbitals as opposed to the three p orbitals in.
Hybridization Chemistry LibreTexts
What Is Hybridization Explain Sp2 Hybridization With Example The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2. Because carbon plays such a significant role in organic chemistry, we will be using it as an. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2. Web what is hybridization? Web the sp2 hybridization occurs when the s orbital is mixed with only two p orbitals as opposed to the three p orbitals in. Web learn the definition of orbital hybridization and the characteristics and geometries of sp, sp2, sp3, sp3d1, and sp3d2 hybridization. Sp 3 hybridization in methane; A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to be linear by vsepr. Web the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to. Understand the types of hybridization, formation of new hybrid orbitals by the. The beryllium atom contains all.
From www.youtube.com
5.2BHybridization Examples sp sp2 sp3 YouTube What Is Hybridization Explain Sp2 Hybridization With Example Web what is hybridization? A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to be linear by vsepr. Sp 3 hybridization in methane; Understand the types of hybridization, formation of new hybrid orbitals by the. The beryllium atom contains all. Web learn the definition of orbital hybridization and the characteristics and geometries of sp, sp2, sp3, sp3d1, and sp3d2 hybridization.. What Is Hybridization Explain Sp2 Hybridization With Example.
From www.youtube.com
How to determine Hybridization s, sp, sp2, and sp3 Organic What Is Hybridization Explain Sp2 Hybridization With Example Understand the types of hybridization, formation of new hybrid orbitals by the. Web the sp2 hybridization occurs when the s orbital is mixed with only two p orbitals as opposed to the three p orbitals in. Sp 3 hybridization in methane; The beryllium atom contains all. A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to be linear by vsepr.. What Is Hybridization Explain Sp2 Hybridization With Example.
From www.pinterest.com
Hybridization Easy Science Chemistry education, Chemistry classroom What Is Hybridization Explain Sp2 Hybridization With Example Web learn the definition of orbital hybridization and the characteristics and geometries of sp, sp2, sp3, sp3d1, and sp3d2 hybridization. The beryllium atom contains all. Understand the types of hybridization, formation of new hybrid orbitals by the. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2. Sp 3. What Is Hybridization Explain Sp2 Hybridization With Example.
From chemistrytalk.org
Hybridization and Hybrid Orbitals ChemTalk What Is Hybridization Explain Sp2 Hybridization With Example Web the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to. Sp 3 hybridization in methane; A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to be linear by vsepr. The valence orbitals of a central atom surrounded by three regions of electron. What Is Hybridization Explain Sp2 Hybridization With Example.
From www.adda247.com
What is Hybridization? sp3, sp2, Examples and Formula What Is Hybridization Explain Sp2 Hybridization With Example The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2. Sp 3 hybridization in methane; Web the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to. The beryllium atom contains all. Web. What Is Hybridization Explain Sp2 Hybridization With Example.
From www.pinterest.com.mx
Orbital Hybridization "Cheat Sheet"? + Example Chemistry lessons What Is Hybridization Explain Sp2 Hybridization With Example The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2. Web the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to. Web learn the definition of orbital hybridization and the characteristics and. What Is Hybridization Explain Sp2 Hybridization With Example.
From www.yourlearningpoint.com
Hybridization, with Example of sp² hybridization Your Learning Point What Is Hybridization Explain Sp2 Hybridization With Example Web what is hybridization? Web learn the definition of orbital hybridization and the characteristics and geometries of sp, sp2, sp3, sp3d1, and sp3d2 hybridization. Web the sp2 hybridization occurs when the s orbital is mixed with only two p orbitals as opposed to the three p orbitals in. Web the sp 2 hybridization is the mixing of one s and. What Is Hybridization Explain Sp2 Hybridization With Example.
From brainly.in
Question 4.24 What is meant by hybridisation of atomic orbitals What Is Hybridization Explain Sp2 Hybridization With Example The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2. Understand the types of hybridization, formation of new hybrid orbitals by the. Web what is hybridization? Web learn the definition of orbital hybridization and the characteristics and geometries of sp, sp2, sp3, sp3d1, and sp3d2 hybridization. A beryllium hydride. What Is Hybridization Explain Sp2 Hybridization With Example.
From ar.inspiredpencil.com
Sp2 Hybridization Examples What Is Hybridization Explain Sp2 Hybridization With Example Web the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to. Understand the types of hybridization, formation of new hybrid orbitals by the. Sp 3 hybridization in methane; Web learn the definition of orbital hybridization and the characteristics and geometries of sp, sp2,. What Is Hybridization Explain Sp2 Hybridization With Example.
From www.animalia-life.club
Sp2 Hybridization Shape What Is Hybridization Explain Sp2 Hybridization With Example The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2. Because carbon plays such a significant role in organic chemistry, we will be using it as an. Web what is hybridization? Web the sp2 hybridization occurs when the s orbital is mixed with only two p orbitals as opposed. What Is Hybridization Explain Sp2 Hybridization With Example.
From www.animalia-life.club
Hybridization Orbitals Chart What Is Hybridization Explain Sp2 Hybridization With Example Web what is hybridization? The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2. Understand the types of hybridization, formation of new hybrid orbitals by the. Web the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron. What Is Hybridization Explain Sp2 Hybridization With Example.
From www.masterorganicchemistry.com
How To Determine Hybridization A Shortcut Master Organic Chemistry What Is Hybridization Explain Sp2 Hybridization With Example Web learn the definition of orbital hybridization and the characteristics and geometries of sp, sp2, sp3, sp3d1, and sp3d2 hybridization. Because carbon plays such a significant role in organic chemistry, we will be using it as an. Sp 3 hybridization in methane; Understand the types of hybridization, formation of new hybrid orbitals by the. A beryllium hydride \(\left( \ce{beh_2} \right)\). What Is Hybridization Explain Sp2 Hybridization With Example.
From cedocdwf.blob.core.windows.net
Explain Sp3D2 Hybridization With An Example at Darlene Gibson blog What Is Hybridization Explain Sp2 Hybridization With Example The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2. Because carbon plays such a significant role in organic chemistry, we will be using it as an. Understand the types of hybridization, formation of new hybrid orbitals by the. A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to. What Is Hybridization Explain Sp2 Hybridization With Example.
From www.youtube.com
How to Determine the Hybridization of an Atom (sp, sp2, sp3, sp3d What Is Hybridization Explain Sp2 Hybridization With Example The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2. Web what is hybridization? The beryllium atom contains all. Web the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to. Sp 3. What Is Hybridization Explain Sp2 Hybridization With Example.
From brainly.in
explain SP2 hybridization with ethene molecule as an example ? Brainly.in What Is Hybridization Explain Sp2 Hybridization With Example Web the sp2 hybridization occurs when the s orbital is mixed with only two p orbitals as opposed to the three p orbitals in. The beryllium atom contains all. Sp 3 hybridization in methane; Web what is hybridization? Web the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one. What Is Hybridization Explain Sp2 Hybridization With Example.
From ppt-online.org
General aspects of chemical structure and reactivity of organic What Is Hybridization Explain Sp2 Hybridization With Example Web learn the definition of orbital hybridization and the characteristics and geometries of sp, sp2, sp3, sp3d1, and sp3d2 hybridization. Web the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to. The valence orbitals of a central atom surrounded by three regions of. What Is Hybridization Explain Sp2 Hybridization With Example.
From www.pinterest.com
Hybridizationsp3,sp2,sp hybridization Hybridization of carbon What Is Hybridization Explain Sp2 Hybridization With Example Web what is hybridization? Web the sp2 hybridization occurs when the s orbital is mixed with only two p orbitals as opposed to the three p orbitals in. Web the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to. Web learn the definition. What Is Hybridization Explain Sp2 Hybridization With Example.
From brainly.in
Explain me about sp sp2 ,sp3 hybridisation with examples Brainly.in What Is Hybridization Explain Sp2 Hybridization With Example Web the sp2 hybridization occurs when the s orbital is mixed with only two p orbitals as opposed to the three p orbitals in. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp2. Understand the types of hybridization, formation of new hybrid orbitals by the. Web the sp. What Is Hybridization Explain Sp2 Hybridization With Example.